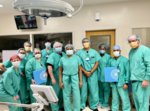
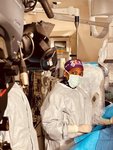
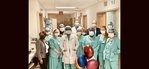
Baptist Health recently broke new ground in the treatment of atrial fibrillation (Afib) as it became the first health system in the Southeast United States to use a new device following its approval by the U.S. Food and Drug Administration (FDA).
Ruby Satpathy, MD, internationally recognized interventional cardiologist with Baptist Heart Specialists and medical director of the Baptist Structural Heart Program, performed the procedure.
The device, Amplatzer Amulet Left Atrial Appendage Occluder, is designed to treat patients with Afib (irregular heartbeats) who are unable to tolerate blood thinners to reduce risk of stroke.
The left atrial appendage (LAA) is a small pouch connected to the upper left chamber of the heart. For people with Afib, it is an area where blood clots can form. If those blood clots reach the bloodstream, they can travel to the brain and cause a stroke.
Following the successful completion of clinical trials in the U.S., the FDA approved the device, which seals the LAA so clots cannot form in this area.
Satpathy implanted the device in Douglas Dixon, 75, from Green Cove Springs. During routine monitoring of his pacemaker, Aaditya Vora, MD, clinical cardiac electrophysiologist, Baptist Heart Specialists, discovered Dixon had experienced an Afib event, though he did not know it.
Having had a brain bleed two years prior, dependence on blood thinners was not a good solution for Dixon. Hence, the referral of Dixon to Satpathy.
“Mr. Dixon was a good candidate for this new device because of his prior brain bleed,” said Satpathy. “The treatment option opens the door for many more patients who cannot be on blood thinners for even a short period. It also allows the LAA closure for different shapes and sizes.”